Unlock smarter training, streamline compliance and simplify audit readiness in life sciences. At Softek Export LLC, we offer a top-notch life sciences learning management system (LMS) tailored to solve the unique challenges of regulated industries like yours. With cutting-edge technology and comprehensive features, our platform ensures seamless processes and regulatory adherence. Take the next step toward effortless compliance and effective training with our reliable ISOtrain.


At Softek Export LLC, we are committed to helping companies organize, validate and maximize their data to meet regulatory requirements.
Our compliance-focused solutions translate training programs into opportunities for advancement and innovation. Be part of the thriving community of life science organizations that have chosen ISOtrain LMS as their trusted ally in training and compliance — and experience the difference in your training processes.
With decades of experience supporting FDA and EMA-regulated organizations, we ensure you achieve your goals through configurable training initiatives and regulatory compliance.
Our learning management system for life sciences is dedicated to helping you adapt your content and structure as your business evolves.
As a global leader in compliance-focused solutions, we are ready to support our global customer base. Count on us for ongoing support and quick issue resolution to keep your operations running.
We have extensive industry experience, allowing us to develop our own systems in-house and meet evolving regulatory standards. We maintain a quality assurance (QA) department to stay on top of industry demands.

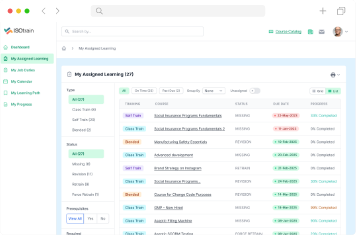


Enhance your training programs, streamline compliance processes and achieve success in the ever-evolving field of life sciences. Our mission is to provide you with a robust solution that simplifies training management, ensures compliance and fosters operational excellence.
Request a demo today to see ISOtrain in action and discover how this system solves compliance problems and empowers your team.