Unlock Your Team’s Potential With ISOtrain
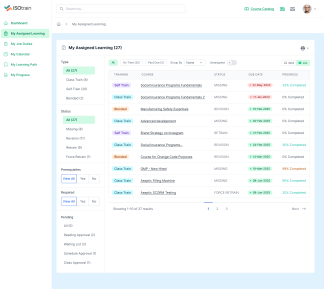
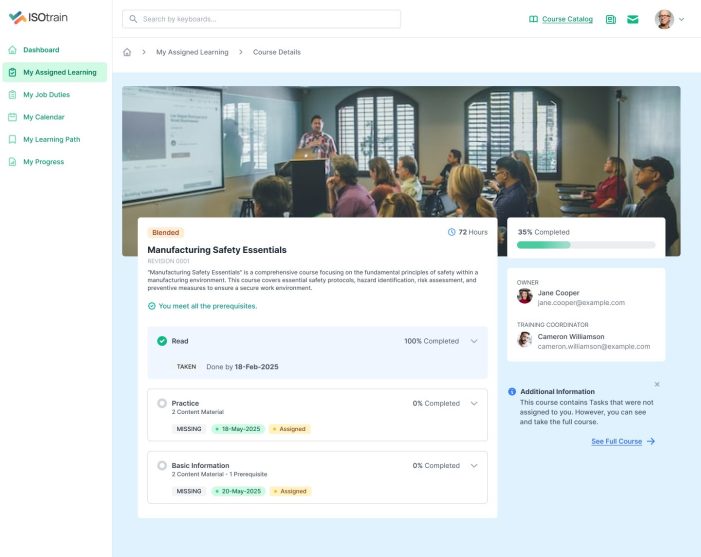
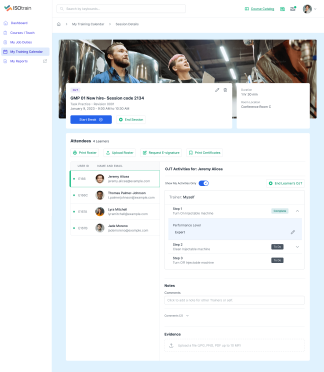
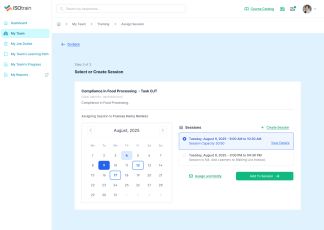
At Softek Export LLC, we’re committed to providing highly regulated industries with an effective, reliable and validated Learning Management System. ISOtrain is the best way to schedule, monitor and update your business’s training process. We ensure your company stays fully compliant with both U.S. and EU-based regulatory requirements, making it perfect for pharmaceuticals, cosmetics, biologics and other industries.
We’re proud to say we’ve been serving various regulated industries for over 25 years. Our knowledge and experience have allowed our software to be in use longer than any other LMS in the market. ISOtrain has provided countless businesses with the essential tools to train their team, analyze qualification statistics and stay up-to-date with compliance regulations.